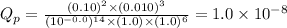Answer:
Value of
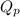 for the given redox reaction is
for the given redox reaction is
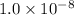
Step-by-step explanation:
Redox reaction with states of species:
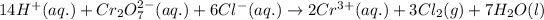
Reaction quotient for this redox reaction:
![Q_(p)=\frac{[Cr^(3+)]^(2).P_{Cl_(2)}^(3)}{[H^(+)]^(14).[Cr_(2)O_(7)^(2-)].[Cl^(-)]^(6)}](https://img.qammunity.org/2020/formulas/chemistry/college/y7wznwbmwl5vquxvo2din08wkrlrvfhd7l.png)
Species inside third braket represent concentration in molarity, P represent pressure in atm and concentration of
 is taken as 1 due to the fact that
is taken as 1 due to the fact that
 is a pure liquid.
is a pure liquid.
![pH=-log[H^(+)]](https://img.qammunity.org/2020/formulas/medicine/college/11uazg5asji4j9glisfdrlkgsy6l390iy3.png)
So,
![[H^(+)]=10^(-pH)](https://img.qammunity.org/2020/formulas/chemistry/college/blvyhewmq38kt479w5cbkxzwkmzadv2in7.png)
Plug in all the given values in the equation of
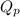 :
: