Answer:
Moles of potassium needed = 36 moles
Step-by-step explanation:
Moles of
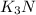 produced = 12.0 mol
produced = 12.0 mol
According to the given reaction:-
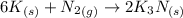
2 moles of
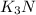 are produced when 6 moles of potassium undergoes reaction.
are produced when 6 moles of potassium undergoes reaction.
Also,
1 mole of
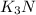 are produced when 6/2 moles of potassium undergoes reaction.
are produced when 6/2 moles of potassium undergoes reaction.
So,
12 mole of
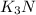 are produced when 3*12 moles of potassium undergoes reaction.
are produced when 3*12 moles of potassium undergoes reaction.
Moles of potassium needed = 36 moles