Step-by-step explanation:
Since, NaCl exists as
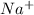 and
and
 in solution. Therefore, Van't Hoff factor (i) will be equal to 2.
in solution. Therefore, Van't Hoff factor (i) will be equal to 2.
Now, we assume that there are "n" moles of NaCl in the given solution. And, we assume that there is 1 kg of solvent (water).
So,
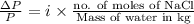
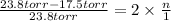
0.264 =
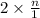
n = 0.132
Also, moles of water will be calculated as follows.
Moles of water =
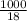
= 55.56 mol
Hence, mole fraction of NaCl is calculated as follows.
Mole fraction =
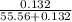
=
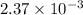
Hence, mole fraction of NaCl will be
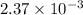 .
.
At
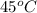 , the vapor pressure will be calculated as follows.
, the vapor pressure will be calculated as follows.
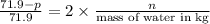
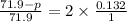
71.9 - p = 18.98
p = 52.92 torr
Therefore, vapor pressure of the given solution is 52.92 torr.