Answer:
-514 kJ/mol
Step-by-step explanation:
The bond enthalpy which is also known as bond energy can be defined as the amount of energy needed to split one mole of the stated bond. The change in enthalpy of a given reaction can be estimated by subtracting the sum of the bond energies of the reactants from the sum of the bond energies of the products.
For the given chemical reaction, the change in enthalpy of the reaction is:
Δ
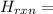 [2(409) + 4(388) + 3(496) - 4(630) - 4(463)] kJ/mol = 818 + 1552 + 1488 - 2520 - 1852 = -514 kJ/mol
[2(409) + 4(388) + 3(496) - 4(630) - 4(463)] kJ/mol = 818 + 1552 + 1488 - 2520 - 1852 = -514 kJ/mol