Answer: The molecular formulas are written below.
Step-by-step explanation:
Saturated hydrocarbons are defined as the hydrocarbons in which a single bond is present between carbon and carbon atoms. The general formula for these hydrocarbons is
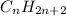
These hydrocarbons have the name ending with the suffix '-ane'
Unsaturated hydrocarbons are defined as the hydrocarbons which have double or triple covalent C-C bonds. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is
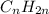 and
and
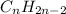
The hydrocarbons having double and triple bonds have the name ending with the suffix '-ene' and '-yne' respectively.
For the given options:
Option a: Hexane
'Hex' prefix is added for the chain having 6-Carbon atoms. So, the formula for this saturated hydrocarbon is
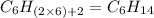
Option b: Methane
'Meth' prefix is added for the chain having 1-Carbon atom. So, the formula for this saturated hydrocarbon is
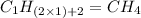
Option c: Propyne
'Prop' prefix is added for the chain having 3-Carbon atoms. So, the formula for this unsaturated hydrocarbon is
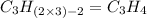
Option d: 1-pentene
'Pent' prefix is added for the chain having 5-Carbon atoms. So, the formula for this unsaturated hydrocarbon is
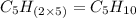 . The double bond is present between C-1 and C-2 atoms
. The double bond is present between C-1 and C-2 atoms
Option e: Octyne
'Oct' prefix is added for the chain having 8-Carbon atoms. So, the formula for this unsaturated hydrocarbon is
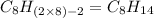
Option f: Decane
'Dec' prefix is added for the chain having 10-Carbon atoms. So, the formula for this saturated hydrocarbon is
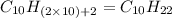
Option g: Heptane
'Hept' prefix is added for the chain having 7-Carbon atoms. So, the formula for this saturated hydrocarbon is
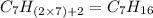
Option h: 4-nonene
'Nona' prefix is added for the chain having 9-Carbon atoms. So, the formula for this unsaturated hydrocarbon is
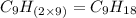 . The double bond is present between C-4 and C-5 atoms
. The double bond is present between C-4 and C-5 atoms