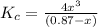Answer : The equilibrium constant expression will be
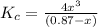
Solution :
Equilibrium constant : It is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
The given equilibrium reaction is,

Initially conc. 0.87 0 0
At equilibrium (0.87-x) 2x x
The expression of
 will be,
will be,
![K_c=([A]^2[B])/([A_2B])](https://img.qammunity.org/2020/formulas/chemistry/college/b2sy1c3w3o833exgg1lfs8a3ibnh24inny.png)
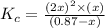
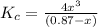
Therefore, the equilibrium constant expression will be