Answer:
The heat transferred from water to skin is 6913.5 J.
Step-by-step explanation:
Given that,
Weight of water = 25.0 g
Suppose that water and steam, initially at 100°C, are cooled down to skin temperature, 34°C, when they come in contact with your skin. Assume that the steam condenses extremely fast. We will further assume a constant specific heat capacity c=4190 J/(kg°K) for both liquid water and steam.
We need to calculate the heat transferred from water to skin
Using formula for stream
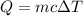
Put the value into the formula
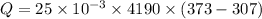
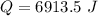
Hence, The heat transferred from water to skin is 6913.5 J.