Answer:
![\pi _(rms)=656.77m/s</strong></p><p><strong>Explanation: </strong></p><p>the following formula can be used for deriving the root mean square velocity</p><p>[tex]\pi _(rms) =\sqrt({3RT)/M}]()
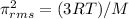
For te Ne as
T=76°C=76+273=349K
molecular mass of Ne=20.18g/mol=0.02018kg/mol
R=8.314J/K.mol ..the gas constant
using the formula
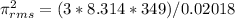
431355.69 j/kg
J=1kg
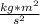 /(kg)
/(kg)
[tex]\pi^2 _{rms}=m^2/s^2
[tex]\pi _{rms}=m/s
therefore, taking the square root of 431355.69 j/kg
[tex]\pi _{rms}=656.77m/s