Answer:
16.5 atm
Step-by-step explanation:
A mixture of He, N₂, and Ar has a pressure of 24.1 atm at 28.0 °C. If the partial pressure of He is 3013 torr and that of Ar is 2737 mm Hg, what is the partial pressure of N₂?
The total pressure of a gaseous mixture is equal to the sum of the partial pressures.
P = pHe + pN₂ + pAr
pN₂ = P - pHe - pAr [1]
We need to express pHe and pAr in atm.
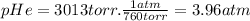
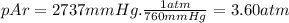
From [1],
pN₂ = 24.1 atm - 3.96 atm - 3.60 atm = 16.5 atm