Step-by-step explanation:
According to the electrochemical series, more positive is the
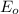 value then more will be tendency of the oxidized form to get reduced by accepting electrons.
value then more will be tendency of the oxidized form to get reduced by accepting electrons.
Similarly, more negative is the value of
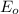 then more will be tendency of the reduced form to get oxidized by donating electrons.
then more will be tendency of the reduced form to get oxidized by donating electrons.
In the given situation,
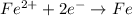 ,
,
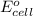 = -0.44
= -0.44
Therefore, we can conclude that metal which spontaneously gets reduced by iron metal is
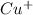 and Ag as these have positive
and Ag as these have positive
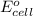 values.
values.