Answer:
Kb = 0.159 °C.kg/mol
Step-by-step explanation:
The condensation point has the same value than the boiling point. The elevation in the boiling point can be calculated using the following expression.
ΔT = Kb × b
where,
ΔT: elevation in the boiling point
Kb: molal boiling point elevation constant
b: molality of the solute
The molality of the urea is:
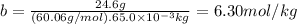
Then,
ΔT = Kb × b
(124.3°C-123.3°C) = Kb × (6.30 mol/kg)
Kb = 0.159 °C.kg/mol