Answer: The volume of solution containing the given amount of glucose is 0.81 L
Step-by-step explanation:
We are given:
8.0 % (m/v) glucose solution
This means that 8 grams of glucose is present in 100 mL of solution
To calculate the volume of solution containing 65 g of glucose, we use unitary method:
8 grams of glucose is present in 100 mL of solution.
So, 65 grams of glucose will be present in
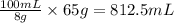 of solution
of solution
Converting this into liters, we use the conversion factor:
1 L = 1000 mL
So,
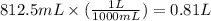
Hence, the volume of solution containing the given amount of glucose is 0.81 L