Answer: 8117 grams
Step-by-step explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
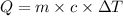
Q = Heat absorbed= 2.50 MJ =
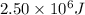
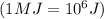
m= mass of substance = ?
c = specific heat capacity = 30.8 J/Kmol
Change in temperature ,
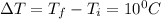
Putting in the values, we get:
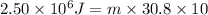
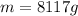
8117 grams of liquid sodium are needed to absorb 2.50 MJ of energy in the form of heat if the temperature of the sodium is not to increase by more than 10.0 °C.