Answer:
The temperature of the cold sink would be 191.1 K
Step-by-step explanation:
The steam engine has a cold and hot reservoir;
Given that heat withdrawn from the source
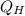 = 10 KJ
= 10 KJ
Work done W= 3 KJ
The hot source temperature
 = 273 K
= 273 K
The efficiency of a steam engine with a hot and cold reservoir can be obtained with equations 1 and 2.
η = W/
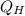 ...................1
...................1
η =
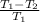 ......................2
......................2
since the two equations represent the efficiency of a steam turbine, equations 1 and 2 are equal.
Eqn 1 = Eqn 2
W/
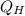 =
=
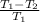 .
.
Substituting the values in equation above to get
 ;
;
3/10 = (273 -
 )/273
)/273
2370 - 10
 = 3 x 273
= 3 x 273
10
 = 2370 -816
= 2370 -816
 = 1911 / 10
= 1911 / 10
 = 191.1 K
= 191.1 K
Therefore the temperature of the cold sink is 191.1 K