Answer :
(a) The mass percentage is 0.0665 %
(b) The concentration in ppm is 665 ppm.
Explanation :
Part (a) :
As we are given that, 0.0350 M aqueous solution of fluoride ion that means 0.0350 moles of fluoride ion present in 1 L of solution.
First we have to calculate the mass of fluoride ion.
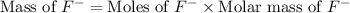
Molar mass of
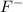 = 19 g/mole
= 19 g/mole
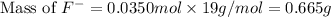
Now we have to calculate the mass of solution.
Mass of solution = Density of solution × Volume of solution
Density of solution = 1.00 g/mL
Volume of solution = 1 L = 1000 mL
Mass of solution = 1.00 g/mL × 1000 mL
Mass of solution = 1000 g
Now we have to calculate the mass -percentage.
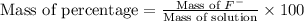
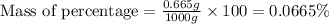
Thus, the mass percentage is 0.0665 %
Part (b) :
Parts per million (ppm) : It is defined as the mass of a solute present in one million
 parts by the mass of the solution.
parts by the mass of the solution.
Now we have to calculate the concentration in ppm.
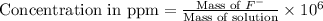
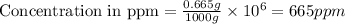
Thus, the concentration in ppm is 665 ppm.