In order to solve this problem, it is necessary to apply the concepts related to density, as a function of volume and mass, as well as the principle of calorimetry.
By definition we know that Density is given as a function of mass in a given volume, mathematically it can be expressed as
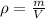
Where,
m = mass
V = Volume
Re-arrange to find the respective mass at each state we have
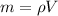
For state 1 we have that the mass is
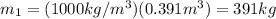
For state 2
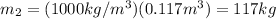
Now from calorimetry we know that heat change is given under
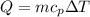
For energy conservation then,
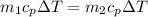
Since the specific heat is the same for the fluid then,
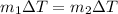
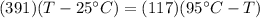
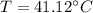
Therefore the final temperature of the mixture will be 41.12°C