Answer : The volume of water produced by this reaction would be, 4.75 liters.
Explanation : Given,
Volume of HCl gas = 9.5 L
The balanced chemical reaction will be:
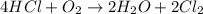
As we know that at STP, 1 mole of gas occupy 22.4 L volume of gas.
By the stoichiometry we can say that:
As, 4 liter volume of HCl gas react to give 2 liter volume of water
So, 9.5 liter volume of HCl gas react to give
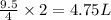 volume of water
volume of water
Therefore, the volume of water produced by this reaction would be, 4.75 liters.