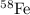Answer : The correct option is, (C)
Explanation :
Neutron capture : In this decay process, an atomic nucleus and one or more number of neutrons collide and combine to form a heavier nucleus. The mass number changes in this process.
The neutron capture equation is represented as,
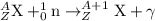
(A is the atomic mass number and Z is the atomic number)
Beta emission or beta minus decay : It is a type of decay process, in which a neutrons gets converted to proton, an electron and anti-neutrino. In this the atomic mass number remains same.
The beta minus decay equation is represented as,
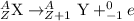
(A is the atomic mass number and Z is the atomic number)
As per question, the cobalt-60 is produced by a three reaction process involving neutron capture, beta-emission, and neutron capture.
Process 1 : Neutron capture.
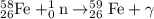
Process 2 : Beta emission.
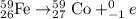
Process 3 : Neutron capture.
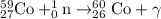
From this we conclude that, the initial reactant in the production of cobalt-60 is
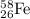
Hence, the correct option is, (C)