Answer:
(a) Homogeneous. 4.7 g of MgCl₂.
(b) 9.1 g
Step-by-step explanation:
(a)
At 200°C, we can dissolve 54.6g of MgCl₂ in 100 g of water. The mass that we could dissolve in 38.2 g of water is:
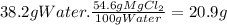
Since we can dissolve up to 20.9 g of MgCl₂ and we added only 16.2 g, the mixture is homogeneous and we could add 20.9 g -16.2 g = 4.7 g of solute to make it saturated.
(b)
At 800°C, we can dissolve 66.1 g of MgCl₂ in 100 g of water. The mass that we could dissolve in 38.2 g of water is:
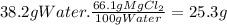
Since we can dissolve up to 25.3 g of MgCl₂ and we added only 16.2 g, we could add 25.3 g - 16.2 g = 9.1 g of solute to make it saturated.