Answer: The molar mass of the saccharin is 181 g/mol
Step-by-step explanation:
We are given:
pH of the solution = 12.190
To calculate pOH of the solution, we use the equation:
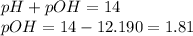
To calculate the
 concentration, we use the equation:
concentration, we use the equation:
![pOH=-\log[OH^-]\\\\1.81=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/c4vggn0knntz9yrdz8ujsch8yxzi5rc50q.png)
![[OH^-]=0.0155M](https://img.qammunity.org/2020/formulas/chemistry/college/aybtym0pbuw0rti3365mkxc4mqf0iyoo9x.png)
As, saccharin is a weak base. Let us consider be as BOH
The equation for the reaction of BOH with water follows:
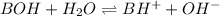
Initial: c
At eqllm: c-x x x
Value of x =
![[OH^-]=0.0155M](https://img.qammunity.org/2020/formulas/chemistry/college/aybtym0pbuw0rti3365mkxc4mqf0iyoo9x.png)
The expression of
 for above equation follows:
for above equation follows:
![K_b=([BH^+]* [OH^-])/([BOH])](https://img.qammunity.org/2020/formulas/chemistry/college/mgolh3iop1j1jzglh8ze4lt8cph98xfh8u.png)
We are given:
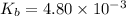
![[BH^+]=0.0155M](https://img.qammunity.org/2020/formulas/chemistry/college/2mr2zivdyskqq1whlyt8j4859omu25tfwe.png)
![[OH^-]=0.0155M](https://img.qammunity.org/2020/formulas/chemistry/college/aybtym0pbuw0rti3365mkxc4mqf0iyoo9x.png)
![[BOH]=c-0.0155](https://img.qammunity.org/2020/formulas/chemistry/college/u6mvyl847ty502d77o0jlvan4ezl7z1trb.png)
Putting values in above expression, we get:
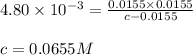
Concentration of saccharin = 0.0655 M
To calculate the molecular mass of saccharin, we use the equation used to calculate the molarity of solution:
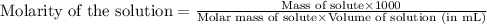
We are given:
Molarity of solution = 0.0655 M
Given mass of saccharin = 0.297 g
Volume of solution = 25.0 mL
Putting values in above equation, we get:
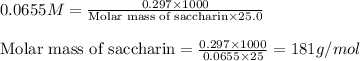
Hence, the molar mass of the saccharin is 181 g/mol