Answer:
50,8 kg of H₂SO₄
Step-by-step explanation:
Moles in 1,16x10⁴L of sulfur dioxide at STP can be obtained using:
n = PV/RT
Where pressure is 1 atm and temperature is 273,15K at STP, knowing R: 0,082atmL/molK:
n = 1atm* 1,16x10⁴L/0,082atmL/molK*273,15K
n = 518 moles
As 1 mol of SO₃(g) produce 1 mol of H₂SO₄(aq), moles of H₂SO₄(aq) can result from the combustion of each metric ton of coal are 518 moles, in grams:
518 moles of H₂SO₄(aq)×
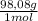 = 50805g = 50,8 kg of H₂SO₄
= 50805g = 50,8 kg of H₂SO₄
I hope it helps!