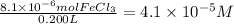Answer:
4.1 × 10⁻⁵ M
Step-by-step explanation:
Let's consider the following balanced equation.
FeCl₃(aq) + 3 AgNO₃(aq) → 3 AgCl(s) + Fe(NO₃)₃(aq)
We can establish the following relations:
- The molar mass of AgCl is 143.32 g/mol.
- The molar ratio of AgCl to FeCl₃ is 3:1.
When 3.5 mg of AgCl are collected, the moles of of FeCl₃ that reacted are:
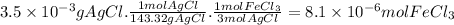
The sample has a volume of 200 mL (0.200 L). The molar concentration of FeCl₃ is: