Answer:
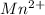 : - F .
: - F .
![[Ar]3d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/o43eoxamxi0a53htschfrjf4y03ihun80u.png)
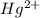 : - G.
: - G.
![[Xe]4f^(14)5d^(10)](https://img.qammunity.org/2020/formulas/chemistry/college/ppew2q2ilpc1pb4t237uxp8wot0vdkrg9t.png)
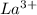 : - D.
: - D.
![[Xe]](https://img.qammunity.org/2020/formulas/chemistry/college/jonww7epfzsmmrzfgjdcypya2igingm0ez.png)
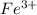 : - F.
: - F.
![[Ar]3d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/o43eoxamxi0a53htschfrjf4y03ihun80u.png)
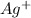 : - C.
: - C.
![[Kr]4d^(10)](https://img.qammunity.org/2020/formulas/chemistry/college/10rjitsz3zd9owmw399rletxn93z0t0sac.png)
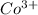 : - E.
: - E.
![[Ar]3d^(6)](https://img.qammunity.org/2020/formulas/chemistry/college/vdwk0y48q0t2390t0kwni4nk1uum72hmg1.png)
Step-by-step explanation:
The electronic configuration of the element Mn is:-
![[Ar]3d^(5)4s^2](https://img.qammunity.org/2020/formulas/chemistry/college/8lse858sf3oikzgvg02d1dcz87ngu2mka8.png)
For,
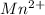 , 2 electrons are lost, thus the configuration is:-
, 2 electrons are lost, thus the configuration is:-
![[Ar]3d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/o43eoxamxi0a53htschfrjf4y03ihun80u.png)
The electronic configuration of the element Hg is:-
![[Xe]4f^(14)5d^(10)6s^2](https://img.qammunity.org/2020/formulas/chemistry/college/7011f4v0sou0rkkjm41xz8njaarkhbvjcr.png)
For,
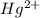 , 2 electrons are lost, thus the configuration is:-
, 2 electrons are lost, thus the configuration is:-
![[Xe]4f^(14)5d^(10)](https://img.qammunity.org/2020/formulas/chemistry/college/ppew2q2ilpc1pb4t237uxp8wot0vdkrg9t.png)
The electronic configuration of the element La is:-
![[Xe]5d^(1)6s^2](https://img.qammunity.org/2020/formulas/chemistry/college/fl0s4gfyt33n9batqxxbiw1xbcbxgl3wj8.png)
For,
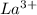 , 3 electrons are lost, thus the configuration is:-
, 3 electrons are lost, thus the configuration is:-
![[Xe]](https://img.qammunity.org/2020/formulas/chemistry/college/jonww7epfzsmmrzfgjdcypya2igingm0ez.png)
The electronic configuration of the element Fe is:-
![[Ar]3d^(6)4s^2](https://img.qammunity.org/2020/formulas/chemistry/college/q6r4pb6a1kigopazivrgxy1nxpfb07v5wo.png)
For,
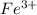 , 3 electrons are lost, thus the configuration is:-
, 3 electrons are lost, thus the configuration is:-
![[Ar]3d^(5)](https://img.qammunity.org/2020/formulas/chemistry/college/o43eoxamxi0a53htschfrjf4y03ihun80u.png)
The electronic configuration of the element Ag is:-
![[Kr]4d^(10)5s^1](https://img.qammunity.org/2020/formulas/chemistry/college/ll3c2d117i7nacj29xpkm3qx86wl9w87xo.png)
For,
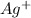 , 1 electron is lost, thus the configuration is:-
, 1 electron is lost, thus the configuration is:-
![[Kr]4d^(10)](https://img.qammunity.org/2020/formulas/chemistry/college/10rjitsz3zd9owmw399rletxn93z0t0sac.png)
The electronic configuration of the element Co is:-
![[Ar]3d^(7)4s^2](https://img.qammunity.org/2020/formulas/chemistry/college/9l94ssmy0c550bopmfjvgtfuj2oi9z2krk.png)
For,
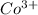 , 3 electrons are lost, thus the configuration is:-
, 3 electrons are lost, thus the configuration is:-
![[Ar]3d^(6)](https://img.qammunity.org/2020/formulas/chemistry/college/vdwk0y48q0t2390t0kwni4nk1uum72hmg1.png)