Answer:
There are 4 single bonds and 2 double bonds.
Step-by-step explanation:
Sulfur atom has six electrons in its outermost shell which can participate in bonding and hence it will form six bonds in total.The chemical formula of sulfuric acid is given by
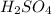 .In sulfuric acid we have four oxygen atoms and two hydrogen atoms.
.In sulfuric acid we have four oxygen atoms and two hydrogen atoms.
Sulfur atom is bonded directly to all the four oxygen atoms out of which two are double bonds.The single-bonded oxygen atoms are inturn bonded to the two hydrogen atoms.
The above statements will make sense after you go through the figure given below.
Hence it has four single bonds and two double bonds.(counting only those bonds in which sulfur is involved)