Answer:
The correct order of increasing vapor pressure: Z,Y,X
Step-by-step explanation:
It is given that the normal boiling point order of three liquids is:
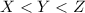
Normal Boiling point is defined as the temperature at which the vapor pressure becomes equal to the atmospheric pressure.
Vapor pressure is the pressure exerted by vapor on the surface of liquid.Hence it increases with tendency of vapor formation.
High boiling point implies that high energy is required to convert the liquid into vapor state and hence the vapor pressure reaches atmospheric pressure at higher temperatures.
Low boiling point implies that the liquid gets easily boiled at a low temperature and converts into vapor with ease and hence exert high vapor pressure.
Bottom line is vapor pressure decreases as boiling point increases at STP.
Hence the order of increasing vapor pressure is Z,Y,X