Answer:
The vapor pressure of water above this mixture is 5.6173 mm Hg.
Step-by-step explanation:
We need to find the vapour pressure due to water in the solution.
According to Raoult's law ,
The vapour pressure due to any one component alone is the product of it's pure vapour pressure and it's mole fraction in the solution .
i.e ,
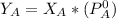
where ,
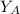 is the partial vapour pressure ( mm Hg)
is the partial vapour pressure ( mm Hg)
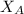 is the mole fraction
is the mole fraction
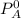 is the pure vapour pressure = 12.8 mm Hg
is the pure vapour pressure = 12.8 mm Hg
∴
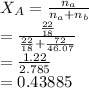
∴
[tex]Y_A=0.43885*12.8\\Y_A=5.6173 mm Hg[tex]
The vapor pressure of water above this mixture is 5.6173 mm Hg.