Answer:
a.
The molarity of Co³⁺ is 0.027 M
The molarity of Cl⁻ 0.081 M
b.
The molarity of Ni³⁺ is 0.022 M
The molarity of SO₄²⁻ is 0.033 M
c.
The molarity of Na⁺ is 0.031 M
The molarity of MnO₄⁻ is 0.031 M
d.
The molarity of Fe²⁺ is 0.021 M
The molarity of Br⁻ is 0.042 M
Step-by-step explanation:
The molarity of each ion is the molarity of the salt times the number of ions in the formula. First, we will calculate the molarity of each salt.
a. Cobalt(III) chloride . CoCl₃
The molar mass of CoCl₃ is 165.29 g/mol. The molarity of CoCl₃ is:
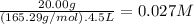
The molarity of Co³⁺ is 1 × 0.027 M = 0.027 M
The molarity of Cl⁻ is 3 × 0.027 M = 0.081 M
b. Nickel(III) sulfate . Ni₂(SO₄)₃
The molar mass of Ni₂(SO₄)₃ is 405.57 g/mol. The molarity of Ni₂(SO₄)₃ is:
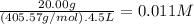
The molarity of Ni³⁺ is 2 × 0.011 M = 0.022 M
The molarity of SO₄²⁻ is 3 × 0.011 M = 0.033 M
c. Sodium permanganate . NaMnO₄
The molar mass of NaMnO₄ is 141.92 g/mol. The molarity of NaMnO₄ is:
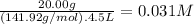
The molarity of Na⁺ is 1 × 0.031 M = 0.031 M
The molarity of MnO₄⁻ is 1 × 0.031 M = 0.031 M
d. Iron(II) bromide. FeBr₂
The molar mass of FeBr₂ is 215.65 g/mol. The molarity of FeBr₂ is:
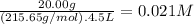
The molarity of Fe²⁺ is 1 × 0.021 M = 0.021 M
The molarity of Br⁻ is 2 × 0.021 M = 0.042 M