Answer : The thermal energy produced during the complete combustion of one mole of cymene is -7193 kJ/mole
Explanation :
First we have to calculate the heat released by the combustion.
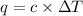
where,
q = heat released = ?
 = specific heat of calorimeter =
= specific heat of calorimeter =
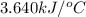
 = change in temperature =
= change in temperature =
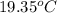
Now put all the given values in the above formula, we get:
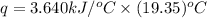
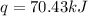
Thus, the heat released by the combustion = 70.43 kJ
Now we have to calculate the molar enthalpy combustion.
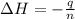
where,
 = molar enthalpy combustion = ?
= molar enthalpy combustion = ?
q = heat released = 70.43 kJ
n = number of moles cymene =
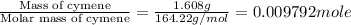
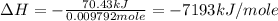
Therefore, the thermal energy produced during the complete combustion of one mole of cymene is -7193 kJ/mole