Answer:
2.02 g
Step-by-step explanation:
Molality (m) is the number of moles of solute dissolved in 1 kg (1000 g) of solvent- that is,
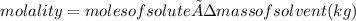
Our first step is to calculate the moles of NH₃ in 475 grams of methanol, using molality as a conversion factor.
0.250 mol/kg × 0.475 kg = 0.119 mol
This solution contains 0.119 moles of NH₃. Now we must convert the moles to grams, using the molar mass of NH₃ as a conversion factor .
17.031 g/mol × 0.119 mol = 2.02 g
Therefore, 2.02 grams of NH₃ must be dissolved in 475 grams of methanol to make a 0.250 m solution.