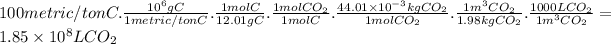Answer:
1.85 × 10⁸ L
Step-by-step explanation:
Coal power plants burn large amounts of coal, C(s), in an O₂(g) atmosphere to generate electricity. The chemical reaction responsible for producing this energy is shown below:
C(s) + O₂(g) → CO₂(g)
Determine the volume of CO₂ in liters produced when 100 metric ton of C(s) is completely burned in an O₂ atmosphere. The density of CO₂ is 1.98 kg/m³ (1 metric ton = 1000 kg: 1 m³ = 1000 L)
We can establish the following relations:
- 1 metric ton = 1000 kg
- 1 kg = 1000 g
- The molar mass of C(s) is 12.01 g/mol
- The molar ratio of C(s) to CO₂(g) is 1:1
- The molar mass of CO₂(g) is 44.01 g/mol
- 1.98 kg of CO₂(g) occupy a volume of 1 m³ (density = 1.98 kg/m³)
- 1 m³ = 1000 L
The volume of CO₂ produced when 100 metric ton of C(s) react is: