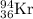Answer: The isotopic symbol that is used to balance the fission reaction is
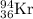
Step-by-step explanation:
In a nuclear reaction, the total mass and total atomic number remains the same.
For the given fission reaction:
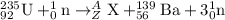
Total mass on reactant side = total mass on product side
235 + 1 = A + 139 + 3
A = 94
Total atomic number on reactant side = total atomic number on product side
92 + 0 = Z + 56 + 0
Z = 36
Hence, the isotopic symbol that is used to balance the fission reaction is