Answer:
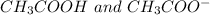
Step-by-step explanation:
Acids are the species which furnish hydrogen ions in the solution or is capable of forming bonds with electron pair species as they are electron deficient species.
When an acid donates a proton, it changes into a base which is known as its conjugate base.
Bases are the species which furnish hydroxide ions in the solution or is capable of forming bonds with electron deficient species as they are electron rich species. When a base accepts a proton, it changes into a acid which is known as its conjugate acid.
The acid and the base which is only differ by absence or presence of the proton are known as acid conjugate base pair.
Thus,
The conjugate base of the acid,
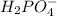 is
is
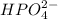
And thus,
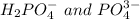 is not a acid conjugate base pair.
is not a acid conjugate base pair.
The conjugate base of the acid,
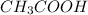 is
is
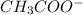
And thus,
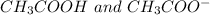 is a acid conjugate base pair.
is a acid conjugate base pair.
The conjugate base of the acid,
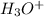 is
is

And thus,
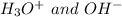 is not a acid conjugate base pair.
is not a acid conjugate base pair.
The conjugate base of the acid,
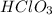 is
is
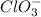
And thus,
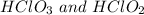 is not a acid conjugate base pair.
is not a acid conjugate base pair.