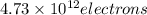Answer : The maximum number of electrons released
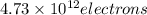
Explanation : Given,
Frequency =
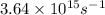
Kinetic energy =
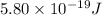
Total energy =
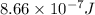
First we have to calculate the work function of the metal.
Formula used :
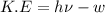
where,
K.E = kinetic energy
h = Planck's constant =
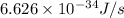
 = frequency
= frequency
w = work function
Now put all the given values in this formula, we get the work function of the metal.
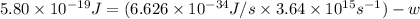
By rearranging the terms, we get
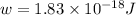
Therefore, the works function of the metal is,
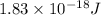
Now we have to calculate the maximum number of electrons released.
The maximum number of electrons released =
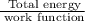
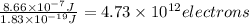
Therefore, the maximum number of electrons released is