Answer:
The volume of
 required is:- 3.75 mL
required is:- 3.75 mL
Step-by-step explanation:
At constant pressure and temperature, the volume of the gases can be used for stoichiometric calculations in terms of moles.
Thus, For the given reaction:-
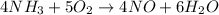
4 mL of
 is required to react with 5 mL of
is required to react with 5 mL of

Also,
1 mL of
 is required to react with 5/4 mL of
is required to react with 5/4 mL of

So,
3.00 mL of
 is required to react with
is required to react with
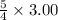 mL of
mL of

The volume of
 required is:- 3.75 mL
required is:- 3.75 mL