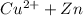Answer:
NONE OF THE ABOVE
Step-by-step explanation:
None of the above are examples of an oxidation - reduction or a redox reaction . This is because there is no change in the oxidation state of any of the elements in the reaction when the reaction happens .
- For a redox reaction , transfer of electrons must take place.
- The oxidation states must increase or decrease in atleast 2 of the elements of a compound.
- For example :
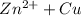 ⇒
⇒