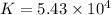Answer:
Part:A
The
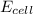 of the reaction is +0.50V
of the reaction is +0.50V
Part-B:
a)
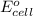 of the reaction is -1.61 V.
of the reaction is -1.61 V.
b)
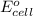 of the reaction is +0.27 V.
of the reaction is +0.27 V.
Part-C:
"K" of the oxidation reaction is
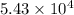 .
.
Step-by-step explanation:
Part:A
The given chemical reaction is as follows.
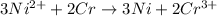
Reduction half reaction:
At cathode:
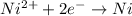
Oxidation half reaction;
At anode:
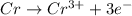
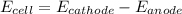
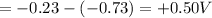
Part-B:
a)
The given chemical reaction is as follows.
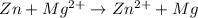
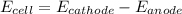
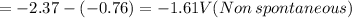
b)
The given chemical reaction is as follows.
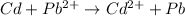
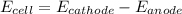
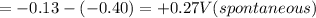
Part-C;
The given chemical reaction is as follows.
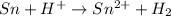
Oxidation half reaction;
At anode:
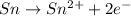
Reduction half reaction;
At cathode:
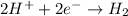
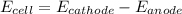
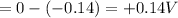
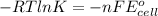
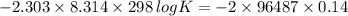
logK = 4.737