he number following the symbol represents a change in neutrons and mass numbers.
Step-by-step explanation:
The chemical symbol is the abbreviation used to identify the element or atom of the element. The isotope symbols of specific element is recorded by placing a mass number as superscript in left to the element's symbol. The protons count (also referred as atomic number) as subscript precedes the symbol. For example, magnesium is the three isotopes mixture with 12 as atomic number and a mass numbers as 24, 25 and 26.
These isotopes can identify as
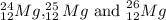 . These isotope symbols are read as "element, mass number" and can be symbolized by this reading. For example,
. These isotope symbols are read as "element, mass number" and can be symbolized by this reading. For example,
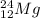 is read as "Magnesium 24" and so on. All magnesium atoms contain 12 protons in the nucleus. So, it indicates the neutron variation and mass numbers.
is read as "Magnesium 24" and so on. All magnesium atoms contain 12 protons in the nucleus. So, it indicates the neutron variation and mass numbers.