Answer:
The total number of valence electrons per atom does not change. It remains at 7 (as opposed to 1 in the options.)
Step-by-step explanation:
The periodic table organizes elements with the same number of valence electrons in the same column.
All the halogens (F, Cl, Br, etc.) are found in the same column (the second column from the right) on the periodic table. The all have the same number of valence electrons per atom.
All elements in the right-most column (the one with all the noble gas elements) contain 8 valence electrons per atom (a full valence shell.) Since the halogens are to the left of the noble gas elements by only one place, each would contain
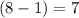 valence electrons.
valence electrons.