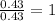Answer:
The molecular formula of this compound is
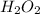
Step-by-step explanation:
From the given,
Mass of compound = 7.36 g
Molar mass of compound = 34.0 g/mol
The mass of oxygen = 6.93 g
The hydrocarbon contains only hydrogen and carbon.
Mass of hydrogen + Mass of oxygen = 7.36 g
Mass of hydrogen + 6.93 g = 7.36 g
Mass of hydrogen = 7.36 - 6.93 = 0.43g
Molar mass of hydrogen = 1.0 g/mol
Molar mass of oxygen = 16 g/mol
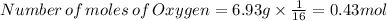
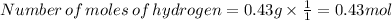
Now divided each one by 0.43
Oxygen:
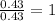
Hydrogen :