Answer:
The amount of sodium in this sample = 32.4 grams
Step-by-step explanation:
Mass of Sodium = 23 g/mol
Mass of Chlorine = 35.5 g/mol
Mass of NaCl = 35.5 g/mol
Fraction of Na in NaCl =

=0.648
We are given a sample of 50 grams of sodium chloride.
The amount of sodium in this sample =
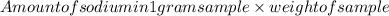
The amount of sodium in this sample =
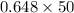
The amount of sodium in this sample = 32.4 grams