Answer:
Final temperature = 821.25 °C
Step-by-step explanation:
In this process, we expand a gas at constant pressure from one temperature to another temperature.
According to Avogadro's law , ratio of volume and temperature for an ideal gas remains constant .
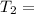 unknown
unknown
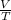 = constant
= constant
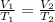
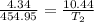
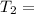 1094.4 K
1094.4 K
To convert the temperature to celsius, subtract 273.15 from final temperature in K.
So ,
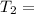 821.25 °C
821.25 °C