Answer:
Copper ( II ) chloride
Step-by-step explanation:
According to the nomenclature ,
- The metal ion name is followed by the non-metal ion
- The oxidation state of the metal is to be mentioned in the brackets .
- As
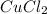 has two
has two
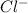 ions , and the net charge on the compound is zero , the charge on copper is :
ions , and the net charge on the compound is zero , the charge on copper is :
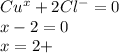
thus ,
⇒
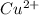
∴ ,
The proper nomenclature is :
Copper ( II ) chloride