Answer:
The weight of ammonium phosphate is 10 gm
Step-by-step explanation:
The chemical reaction of ammonium phosphate formation is as follows.
![3NH_(3)+H_(3)PO_(4) \rightarrow [(NH_(4))_(3)PO_(4)]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ch542r0ah1i4irod08h7qfuzxstn57fy6e.png)
From the reaction one mole of phosphoric acid is equivalent to the one mole of ammonium phosphate.
So, "n" mole of phosphoric acid is equivalent to the "n" mole of ammonium phosphate.
Number of moles in 7.1 grams of phosphoric acid =
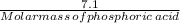
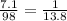
Formation weight of ammonium phosphate :
Weight of ammonium phosphate =
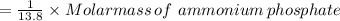
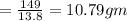
Round the answer to 2 significant digits
Weight of ammonium phosphate = 10 gm