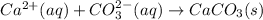Answer:
The net ionic equation:
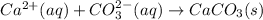
Step-by-step explanation:
The given chemical reaction is as follows.
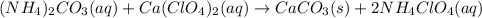
The total ionic equation is as follows.
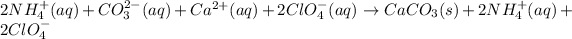
Similar ions on the both sides of the reaction will be cancelled.
The remaining reaction is
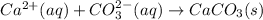
Therefore, the net ionic reaction is as follows.