Answer:
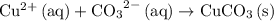 .
.
Step-by-step explanation:
Start by balancing the chemical equation. (In this question, the equation was already balanced.)
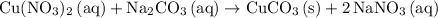 .
.
Go through all the chemicals in the equation. If the chemical is one of the followings, rewrite it as the ions it contains:
- A strong acid,
- A strong base, or
- A soluble salt (look for state symbol
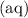 , not
, not
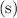 .
.
In this case, all four chemicals are salts. However, only the three of them are soluble:
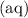 :
:
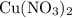 ,
,
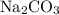 , and
, and
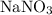 .
.
- Rewrite
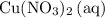 as
as
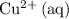 and
and
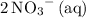 .
. - Rewrite
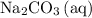 as
as
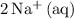 and
and
 .
. - Rewrite
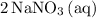 as
as
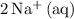 and
and
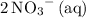 .
.
After rewriting, the chemical equation becomes an ionic equation. However, this equation is not a "net" ionic equation due to all the duplicates.
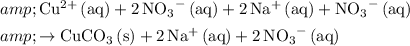 .
.
To obtain the net ionic equation, simply eliminate the duplicates.
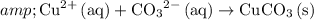 .
.