Answer:
Moles of sulfur hexafluoride gas = 0.428 moles
Mass of sulfur hexafluoride gas = 62.5 g
Step-by-step explanation:
Given that:
Pressure = 0.300 atm
Temperature = 26 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (26 + 273.15) K = 299.15 K
Volume = 35.0 L
Using ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
0.300 atm × 35.0 L = n × 0.0821 L.atm/K.mol × 299.15 K
⇒n = 0.4275 moles
Moles of sulfur hexafluoride gas = 0.428 moles
Molar mass of sulfur hexafluoride gas = 146.06 g/mol
The formula for the calculation of moles is shown below:

Thus,
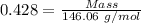
Mass of sulfur hexafluoride gas = 62.5 g