Answer:
0.0366
Step-by-step explanation:
The problem states there is 2.00M of CaCl2. This can be re-written as 2.00 moles of CaCl2 over 1 liter of solution:
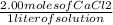
What we want is the mole fraction of CaCl2 which can be written as moles of solute over total moles of solution:
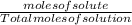
As you can see, the numerator of the first equation matches the numerator of the second equation so we don't need to do anything to solve for the numerator of the second equation. Therefore let's focus on solving the denominator of the second equation.
Start by converting 1 liter of solution into grams:
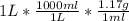 = 1,170 grams of solution
= 1,170 grams of solution
We can use the density provided to us in the problem to convert from volume units to mass units.
So we know that there are 1,170 grams of solution but since we don't know how much of that solution is CaCl2 and how much of that solution is H20, the next step would be to find the mass (in grams) of CaCl2 and then subtract that mass to the total mass of solution. The amount of solution left after subtracting the amount of CaCl2 is the amount of H20 in the solution:
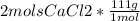 = 221.96g CaCl2
= 221.96g CaCl2
1170g solution - 221.96g CaCl2 = 948.03g H20.
Next step is to convert the grams of H20 into moles:
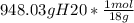 = 52 moles of H20.
= 52 moles of H20.
Now we have all the information we need to plug into the second equation and solve for the mole fraction of CaCl2:
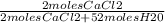 = 0.0366
= 0.0366