Answer:
0.675 atm
513 Torr
Step-by-step explanation:
Given is that, the atmospheric pressure on the surface of Venus is
6.84 X 10⁴ Pa.
1 atm (atmospheric pressure) is equal to 101325 pascal (Pa).
To convert divide the pressure value by 101325.
Pressure in atm =
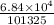
= 0.675055 atm
Rounding it off to 3 significant digits: 0.675 atm
Now, one Torr is 133.322 Pa. For conversion, divide the pressure value by 133.322.
Pressure in Torr =
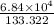
=513.04219 Torr
Rounding it off to 3 significant digits: 513 Torr