Answer:
T2= 1132-273= 859°C
Step-by-step explanation:
The root mean velocity of a molecule is given by
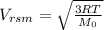
R= gas constant
T= temperature of the gas
Mo= molecular weight of the gas
⇒V_{rsm}∝√T
⇒
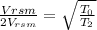
T_0= 273+10=283K
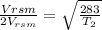
solving we T2= 1132 K
therefore T2= 1132-273= 859°C