Answer:
1.88 g
Step-by-step explanation:
Considering:-
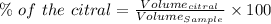
Given that:-
% Citral = 5.72 %
Volume of the citral = 0.120 mL
So,
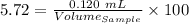
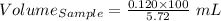
Volume of the grass oil sample = 2.10 mL
Also, Density of the lemon grass oil = 0.893 g/mL
Mass = Density*Volume = 0.893 g/mL*2.10 mL = 1.88 g
1.88 g of lemon grass oil did you begin with if citral comprises 5.72% of the oil.